Ligands
Radioligand therapy (RLT) has long been a cornerstone of cancer treatment, leveraging high-energy radiation to damage the DNA of cancer cells, ultimately leading to cancer cell death. However, traditional radiotherapy can also harm healthy tissues, necessitating the development of more targeted approaches that spare normal cells. Radiopharmaceuticals, which consist of radioactive isotopes attached to a Ligand, or targeting molecules, represent one such advancement. In cancer therapy, the most commonly used ligands can be Small Molecules, Peptides, or Antibodies, each offering distinct advantages in terms of specificity, tissue penetration, and therapeutic efficacy. Below are descriptions of the drug modalities discussed above, as well as typical advantages and disadvantages associated with each.
Comparison of Ligand Modalities
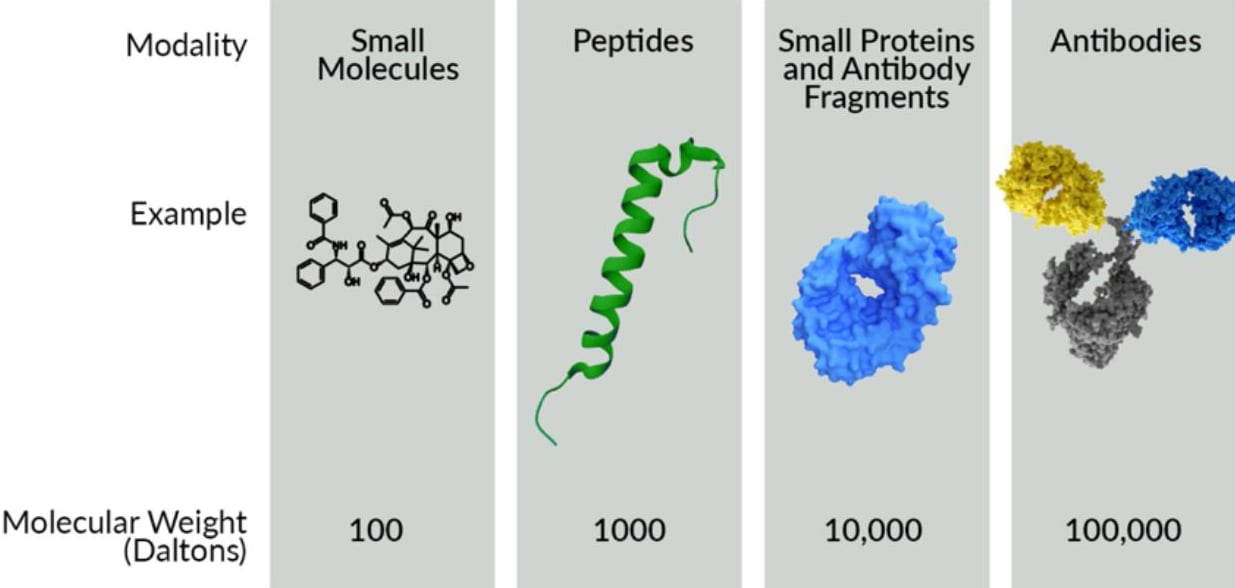
Types of Ligand Molecular Modalities Typically Applied to Radiopharmaceuticals
- Small Molecule Radiotherapy: Small molecule radiopharmaceuticals are compact organic compounds that can be radiolabeled and used for targeted radiotherapy. These compounds can be designed to selectively bind to specific cellular receptors or antigens on cancer cells. Small molecules are advantageous due to their relatively simple structure, the abundance of chemical synthesis methods available to make many versions of a small molecule, their rapid clearance from the body, and their ability to penetrate tissues or bind in less accessible areas (e.g. an enzyme active site). They are commonly used in positron emission tomography (PET) imaging and targeted radionuclide therapy (TRT) for various cancers. While their small size imparts certain advantages, it also is the primary disadvantage of small molecules. The limited size translates to limited interaction with the chosen target and makes it more difficult to gain target specificity, especially in cases where healthy tissue may have highly related proteins expressed as well. Effects of the small molecule drug, or metabolites of the drug on liver toxicity must also be closely monitored.
- Peptide or Small Protein Radiotherapy: Peptide or small protein-based radiopharmaceuticals offer a balance between the small size of small molecules and the high specificity of antibodies. Peptides are short chains of amino acids that can be developed to bind with high affinity to receptors that are often overexpressed in cancer cells, such as somatostatin receptors in neuroendocrine tumors. Small proteins, such as antibody fragments, likewise can be designed for high target specificity, and high affinity. Radiolabeled peptides, like 177Lu-DOTATATE, have shown significant success in clinical settings, providing effective targeting with manageable off-target effects. The moderate size of peptides allows for highly effective tissue penetration and efficient internalization into cells while maintaining a high degree of specificity. Peptide-based radiopharmaceuticals aim to selectively deliver radiation to cancer cells while minimizing damage to healthy tissues. The primary disadvantage of peptide radiotherapies is that the binding partner on the cell must be unique to the cancer cells. If the binding partner is present on healthy cells, the peptide radiotherapy will also irradiate the healthy cells, likely causing undesired side effects. The number of binding partner targets that are unique to, or dramatically overexpressed in cancer cells is limited, and multiple drugs are already available for such targets.
- Antibody Radiotherapy: Monoclonal antibodies (mAbs) are large proteins that specifically recognize and bind to tumor-associated antigens predominantly expressed on cancer cells. They can be designed to be highly specific to a single biological target and can have unmatched affinity to the desired target. Radiolabeled mAbs are used for both imaging and therapy. In radioimmunotherapy, mAbs deliver radiation (usually beta particles) directly to cancer cells. The large size of antibodies, while limiting tissue penetration, provides prolonged circulation times and mAbs can engage the patient’s natural immune system towards the cancer. The extended circulation time and immune system engagement of antibodies is a significant advantage in many drug development settings, but these properties pose problems in the setting of radiotherapy. Prolonged circulation of radioisotopes when conjugated to a mAb results in damage to healthy tissues and may deplete immune cells. Known modifications can be made to decrease the circulation time of mAbs, but peptides or small proteins tend to be preferred in radiotherapy because they have preferred circulation properties. Hence, radiolabeled mAbs are typically limited to low doses in therapy or restricted to imaging applications. As with peptides, monospecific mAbs also bind specifically to a single target and, therefore, suffer from the same target restrictions.
Emerging Technologies: Bispecific and Multi-specific Antibodies in Radiopharmaceuticals
- Cutting-edge innovations in Bispecific (BsAbs) and Multi-specific antibodies (MsAbs) represent a transformative advancement in the field of biotechnology. These groundbreaking molecules are transforming cancer treatment by offering unprecedented precision in targeting diseased cells while avoiding healthy tissue. Bispecific antibodies, which can bind to two different targets simultaneously, and multi-specific antibodies, which can target three or more, are making significant strides in enhancing the efficacy and safety of cancer therapies. This multi-targeting ability has been used to high effect in oncology to expand the effectiveness of an antibody by allowing it to bind multiple targets, or to improve the specificity of the drug to only cells that have two or more cancer targets present.
- In the field of radioimmunotherapeutics, BsAbs and MsAbs are gaining attention as pre-targeting reagents. A pre-targeting reagent is a non-radioactive agent that can be pre-administered to a patient that selectively targets the tumor cells. It includes an additional binding domain that binds to a radioligand that can be administered after the pre-targeting agent. An example of such a pre-targeting agent is Self-Assembling and Disassembling (SADA) bispecific antibodies. The advantage of pre-targeting with an agent like SADA is that a small antibody fragment can be targeted to any tumor antigen, and coupled to a domain that binds to a single radioligand, making the radioligand binder a universal component for any compatible tumor targeting antibody fragment. Additional work to combine the specificity and potency of multi-specific antibodies and radioligands is ongoing, and the results will be exciting to see as new treatments become available.
Summary
The choice between modalities in radiotherapy depends on individual health, tumor characteristics, and treatment goals. The use of small molecules, peptides, and antibodies as targeting agents in radiotherapy offers a spectrum of strategies to enhance the precision and efficacy of cancer treatment. Each modality brings unique strengths to the table, and ongoing research continues to optimize these approaches, aiming to maximize therapeutic efficacy while minimizing adverse effects on healthy tissues. A major shortfall of all the standard modalities listed here is that one drug binds to one target type. The 1:1 nature of the interaction means that target expression in healthy tissues can be a significant safety concern, or loss of expression in the cancer cells renders the drug inactive. Ongoing research into multi-modal drugs (e.g. multi-specific antibodies) holds significant promise for pre-targeting radiotherapies to a broader range of targets and lower the chance that cancer cells may escape the treatment.


