Basics of Theranostics
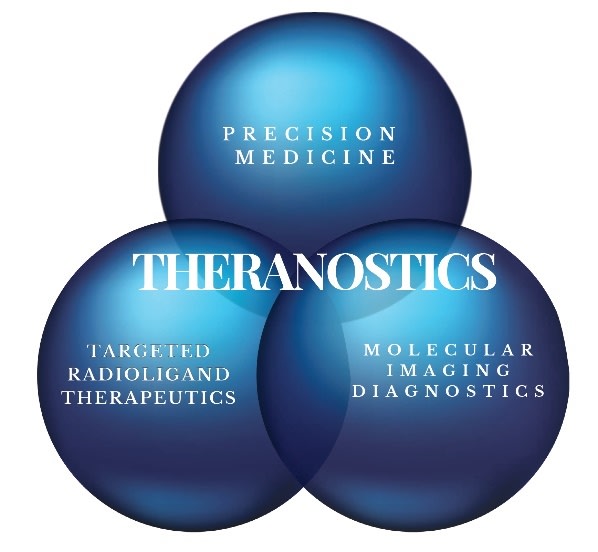
Theranostics is a combination of the terms THERApeutics (Targeted Radioligand Therapeutic) and diagNOSTICS (Molecular Imaging). Theranostics is the term used to describe the combination of a radioisotope that has either imaging properties or therapeutic properties to a construct that is set to only go to a specific target in the body that in this case is targeting a cancer cell (Precision Medicine). If the radioisotope has the properties to locate a cancer cell than this is considered diagnostic molecular Imaging (PET scan). If the radioisotope has the properties to kill a cancer cell than this is considered radioligand therapeutic (RLT). In either case the radioisotope is linked to a Ligand that is set to find a specific target found on a cancer. For Theranostics, the ligand is either an Antibody or a Small Molecule. There are pros and cons to whether an antibody or a small molecule is chosen as the ligand.
Parts of a Theranostic
All theranostics involve the same five components: a ligand, linker, radioisotope, chelator, and a target.
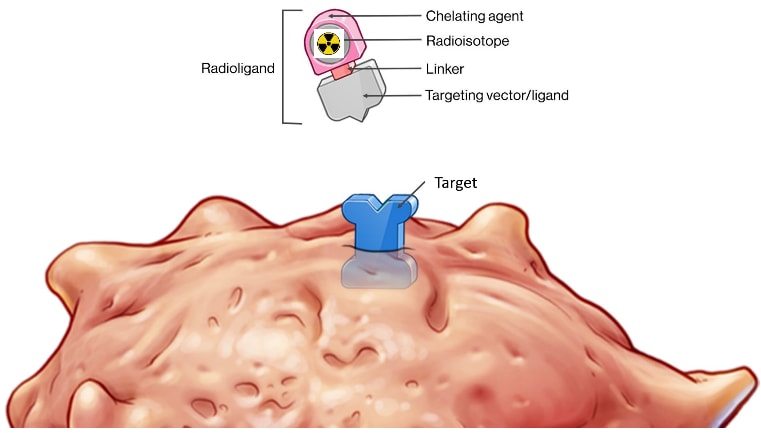
- Ligand: A molecule that binds to a radioisotope and delivers it to a specific site on the surface of a cancer cell, known as the target. The ligand can be either an antibody or a small molecule.
- Linker: This component connects the ligand to the radioisotope, similar to a hitch on a truck. It is crucial for the stability of the radioligand therapy (RLT) while in circulation, preventing the premature release of the radioisotope before it reaches the target and ensuring efficient release at the target site.
- Chelator: This securely holds the radioisotope in place, akin to strap tie-downs on a cargo truck. It is vital to prevent the premature release of the radioisotope and to determine its release at the target site.
- Radioisotope: A radioactive atom undergoing radiation decay to become stable and non-radioactive. Depending on the type of energy released during decay, it can be used for imaging or treating cancer.
- Radioligand Target: The specificity and abundance of the target on the cancer cells determine the effectiveness of detection on PET scans and the success of RLT. A highly specific target reduces side effects, while a less specific target increases the likelihood of side effects. For example, PSMA is a common target for prostate cancer but is also found in salivary glands, leading to potential side effects like dry mouth when targeting prostate cancer.
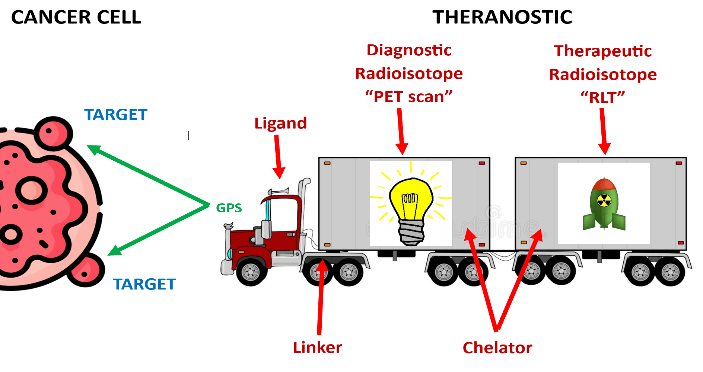
Simple Analogy of a Theranostic
A simplified analogy would be to compare theranostics to a truck hitched to a trailer. The truck represents the ligand, which is set to a specific GPS coordinate—the cancer target. The truck is connected by a linker to a trailer. The trailer is filled with either light bulbs (for imaging) or bombs (for therapeutic purposes). The trailer itself represents the chelator, which holds the payload securely in place. If the payload consists of light bulbs, this setup is used for molecular imaging, such as a PET scan, to identify the location of the cancer. If the payload consists of bombs, it is used for radioligand therapy (RLT) to treat the cancer.
How Are Theranostics Administered
Theranostics, whether for imaging or therapeutic purposes, involve the use of radiation and must be administered by highly trained professionals at approved centers. These specialists are skilled in meticulously handling and safely administering the theranostic agents, as well as providing necessary patient education. Theranostics are typically given via a slow push injection through an IV. After receiving your injection, you usually need to wait for a safe period in a special room called an uptake room, which has protective features such as lead-lined walls. The length of time you spend in the uptake room depends on the specific theranostic agent you received.
Upon discharge, you will receive important instructions to ensure the safety of those around you. For example, after receiving a beta theranostic agent, you may be advised to avoid close contact (closer than 3 feet) for more than 30 minutes at a time for the first three days post-injection. These precautions are extended for children and pregnant women.
In contrast, after an alpha theranostic injection, the alpha radiation, which can only travel a short distance (a few cells), cannot penetrate beyond your skin. This allows for immediate safe contact with others, but you may still receive instructions such as flushing the toilet twice as your urine will be radioactive for a period of time. Always follow the specific instructions provided by your medical team.
Theranistic Clinical Trials
Currently, there are only a few FDA-approved theranostic agents, but several hundred clinical trials are underway to explore new ones. These trials are investigating numerous combinations of ligands, linkers, radioisotopes, chelators, and targets to develop more effective and less toxic theranostics. They are examining the use of theranostic agents alone or in combination with other cancer therapies and evaluating their effectiveness across various cancer stages, from advanced metastatic cancer that has been heavily pre-treated to early-stage cancer that has just recurred after surgery or radiation. Imaging clinical trials are also being conducted at the initial diagnosis of cancer.
It's important to note that different pharmaceutical companies may explore the same target (such as PSMA for prostate cancer) and the same radioisotope (such as Lu177), but the outcomes can vary significantly if different linkers or chelators are used in the theranostic. Each component of a theranostic plays a crucial role in its effectiveness and safety.

