Basics of Radiation

The thought of radiation can be scary and often thought of as something that should be avoided if at all possible. Radiation is often misunderstood and actually has many very safe uses that we use or encounter in our daily activities. The following education is meant to shed light on what radiation is, the different types of radiation, and ways in which we can use it to our benefit.
Radiation is Unavoidable
Simply, radiation energy that travels through space. Radiation is caused by unstable atoms that emit energy as they decay in an attempt to become stable. Radiation may be non-Ionizing, which gives off less energy and unable to destabilize another atom. Examples of non-ionizing include: light, heat, and radio waves. Radiation may also be ionizing, which gives off more energy and is able to destabilize other atoms. is defined as an energy that travels through space. Radiation is naturally occurring and unavoidable. Radiation occurs in the soil, water, and air and even varies from where you live, the materials in your house, to the foods you consume. The amount of radiation exposure is increased living at higher elevations. Radiation exposure is 3-4 times higher living in the mountains of Colorado versus living at sea-level. Radiation exposure is higher living in a stone or brick house versus a home built of wood material. The granite used in many countertops emits radioactivity. People are exposed to radiation flying in an air plane and believe it or not even eating a banana. This is not meant to scare you. Rather this information is meant to educate you that radiation is everywhere and unavoidable yet not as harmful as it is often made out to be. Radiation has several medical uses that have saved or prolonged countless lives. Don’t get me wrong, there are very dangerous forms of radioactivity. Let’s now explore the science of radiation and the different types of radiation.
The Science of Radiation
Radiation is an energy given off by unstable atoms. Atoms are the basic building blocks of all matter. Atoms the basic building block of all matter. It consists of a nucleus that contains neutrons and protons. The atoms are surrounded by electrons in orbit. consist of a nucleus that contain a proton and a neutron. This nucleus is surrounded by electrons in orbit. When atoms are left undisrupted these atoms are stable and non-radioactive. However, when there is disruption or an imbalance in the atom, the atom becomes unstable and the atom starts the process of radioactive decay the process where an unstable atom loses particles which give off energy (radiation) in an attempt to become stable (non-radioactive). The process of decay in an attempt to reset the atom and become stable again. The process of decay gives off various amounts of energy. This energy is called radiation and the atom is considered radioactive and called a radioisotope the unstable for an element that is emitting radiation during its decay mode in an attempt to become stable. Each radioisotope gives off a known amount of radiation and all radioisotopes also have a known constant speed of decay much like a cruise control on a car. This speed of decay is called the half-life (t ½) the amount of time that it takes for half of the radioactive atoms of a radioisotope to decay. Typically, a radioisotope is considered non-radioactive after 10 half-lives.. One half-life is the time it takes for half of the unstable atoms to undergo radioactive decay. Some radioisotopes have a very short half-life such as Carbon-11 which has a 20-minute half-life. Other radioisotopes have a very long half-life, such as Uranium-238 found in the Earth’s crust which has a half-life of 4.5 billion years.
Types of Radiation
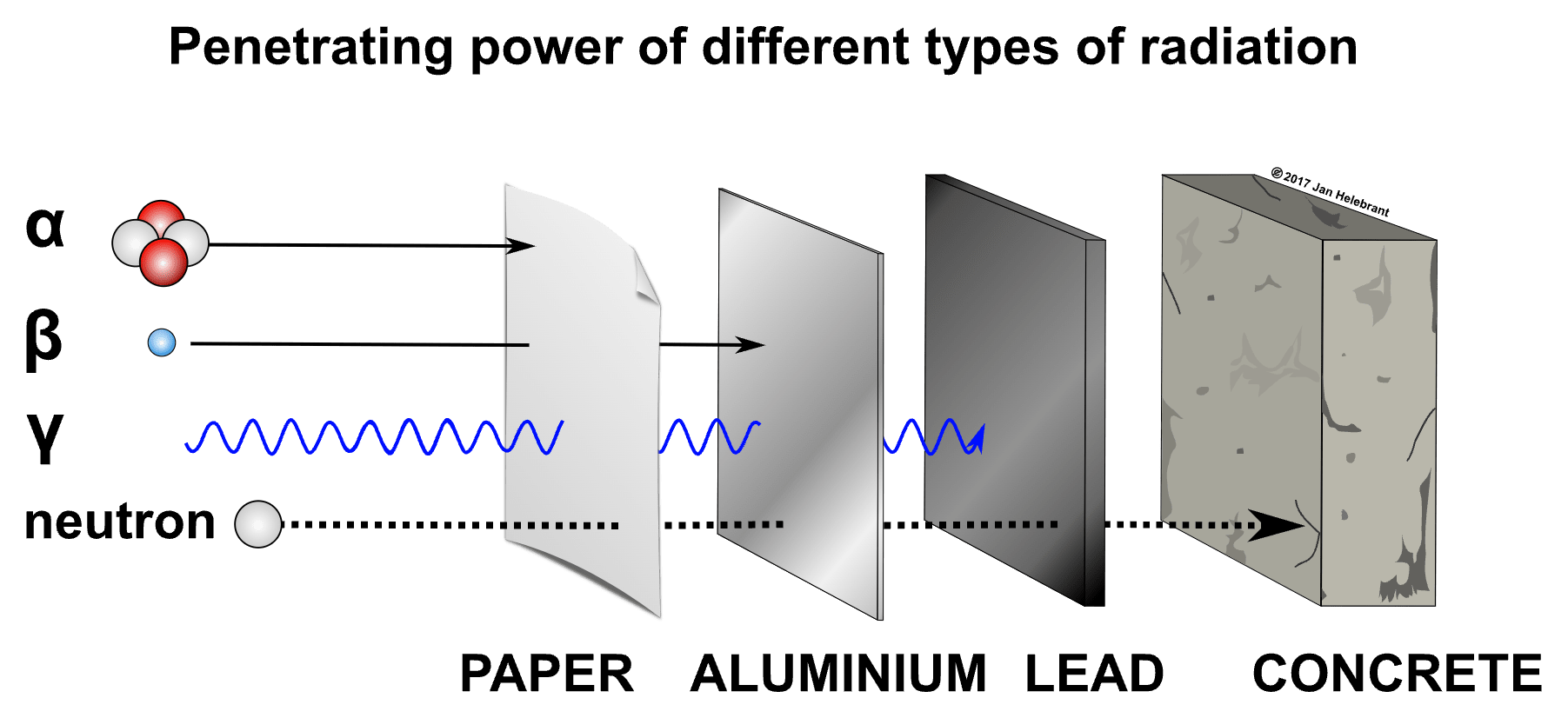
Radiation can be classified as ionizing radiation a type of radiation that emits enough energy that it can make other atoms in its path unstable and radioactive. and non-ionizing radiation a type of radiation that does not emit enough energy to make other atoms in its path unstable or radioactive. Forms of Non-ionizing radiation include: light, microwaves, and radiowaves. . If during the radiation decay process, the energy is high enough that it can make other atoms in its path unstable, this is referred to as ionizing radiation. There are 5 types of Ionizing radiation: Alpha are radioactive particles that are very heavy so alpha particles can only travel a very short distance and they are unable to penetrate most matter. Alpha radiation can’t travel through skin or even a piece of paper. Alpha particles do however emit a high amount of radiation. It only takes 2-10 hits from an alpha particle to damage DNA. Alpha radiation can be used to treat cancers., Beta are radioactive particles that are smaller, travel a greater distance, and can penetrate through a body. Beta radiation emits less energy than alpha. It takes 1,500-2,000 hits from a beta particle to damage DNA. Beta radiation can be used to treat cancers., Neutron Particles a form of ionizing radiation that presents as free neutrons. Typically produced during a phenomena called nuclear fission or nuclear fusion. Neutron radiation is very penetrating. It can take several feet of concrete to stop neutron radiation., Gamma a high energy electromagnetic radiation without mass. Gamma radiation travels a long distance and is commonly used for imaging purposes., and X-rays a high energy electromagnetic radiation without mass. X-Ray radiation travels a long distance and can be used for imaging or treatment of a cancer.. In contrast, non-ionizing radiation is unstable, but gives off much lower levels of energy that are unable to destabilize other atoms. Examples of non-ionizing radiation include visible light, radio waves and microwaves. Non-ionizing forms of radiation carry much less concerns for causing harm to a person.
Medical Uses of Radiation
Ionizing radiation has many medical uses that have saved or prolonged many lives. Such uses include imaging, such as X- rays, CT scans, PET scans; and therapies such as external beam radiation, brachytherapy (seeds), and now the expanding field of theranostics the combination of a radioisotope to a ligand that binds to a specific target on a cancer cell. Theranostics can provide imaging or therapeutic results depending on which radioisotope is used. Theranostics is the combination of 2 words THERApeutic & diagNOSTIC. (Radioligand Therapeutics & Molecular Imaging). Of the different types of ionizing radiation (alpha, beta, neutron particles, gamma, and X-rays), gamma radiation and a type of Beta radiation called Positron Emission are used for imaging cancers. Gamma radiation creates images with the use of a gamma camera (bone scans) or a SPECT CT scan. Positron radiation is imaged with the use of Positron Emission Tomography (PET scan). Alpha and beta radiation are therapeutic and can be used to kill cancer cells. Alpha and Beta radiation have different properties. Alpha radiation is much heavier than Beta radiation by approximately 15,000-fold. Given, alpha radiation is so heavy, it can only travel a very short distance but carries a much higher energy. Alpha radiation only has to hit the DNA of a cancer cell 2-10 times for cell death to occur. On the other hand, beta radiation is much smaller, travels a longer distance which may affect other cells in its path causing extra side effects and has a much lower energy to kill cells. It takes 1500-2000 hits to the DNA to cause cell death.
Measurements of Radiation
There are many units used to measure the amount of radiation may be given as a treatment or to describe the amount of radiation exposure. These include:
Curies/Becquerels (Ci/Bq) are used to describe the DOSE or AMOUNT of radiation that is given. Curies are more widely used in the United States and Becquerels are used more often outside of the United States.
Gray (Gy) is the amount of ionizing radiation that is ABSORBED BY ORGANS AND TISSUES of the body.
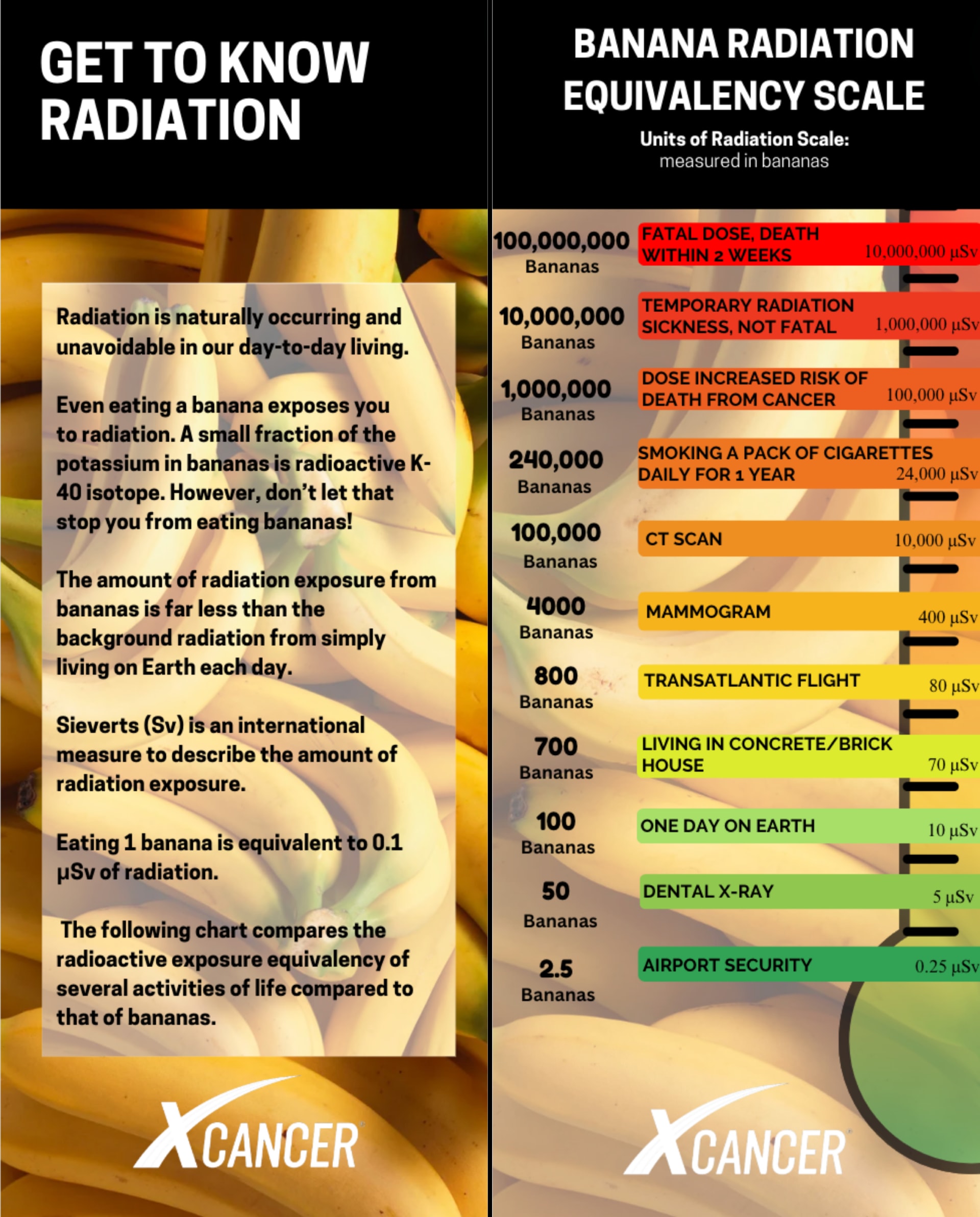
Roentgen Equivalent Man (REM) and Sievert (Sv) measures an EQUIVALENCY OF EXPOSURE. How much radiation a person has been exposed to versus a safe standard. Rems are more commonly used in the United States. Whereas, Sieverts is a more common unit outside of the United States. Even something as simple as eating a banana exposes a person to radiation. Bananas contain potassium. A small portion of the potassium is radioactive potassium-40. Eating one banana is the equivalent to 0.1 micro sieverts of radiation exposure. Please don’t stop eating bananas as they are very healthy and the radioactive exposure is near zero. As you can see in the chart below that compares many of our daily activities which are considered non-harmful by using a banana comparison
Below is a radiation equivalency chart that compares eating bananas to many daily activities or just living on Earth. The intention of this article was to dimmish the fear of radiation and educate on the various types of radiation and how it is safely used to benefit our lives.